Laboratory
Toxicity Identification Evaluation
Feb. 14 2020
Toxicity Identification Evaluation (TIE) is a site‐specific study that isolates, identifies and confirms the causative agents of toxicity in an effluent. TIE is conducted based on procedures promulgated by the United States Environmental Protection Agency (USEPA). Toxicity Reduction Evaluation (TRE) is used as a tool to identify toxic components that may be removed or reduced in an industrial effluent to alleviate toxicity problems. It is usually necessary to complete a TIE before performing the TRE.
TIE uses the responses of test organisms to detect the presence of toxicants before and after samples are subjected to a series of physical and chemical manipulations. This allows one to isolate and subsequently identify the responsible toxicants. The USEPA [1],[2],[3] provides details for this approach including special procedures for municipal wastewater TIEs and TREs [4].
Toxicity may be determined using a battery of freshwater aquatic organisms, which most commonly include: algal growth, Pseudokirschneriella subcapitata; bacterial test, Microtox®, Vibrio fischeri; macroinvertebrate survival, Daphnia magna; and a fish bioassay, fathead minnow, Pimephales promelas or rainbow trout Oncorhynchus mykiss. This battery of aquatic tests measures short‐term toxicity, or acute lethality, and is recommended by Environment Canada for the assessment of water quality [5].
Bureau Veritas can perform TIE for estuarine or marine receiving environments using the following test organisms: algal growth Champia parvula; bacterial test, Microtox® Vibrio fischeri; echinoderm fertilization, Dendraster excentricus; and topsmelt, Atherinops affinis. These methods are described further by the USEPA Marine Toxicity Identification Evaluation Guidance document [6].
Special methods can also be utilised for freshwater and marine sediments using appropriate sediment invertebrate organisms. Hyalella azteca and Chironomus tentans can be used for freshwater sediments. Echinoderm or bivalve embryo tests and marine and estuarine amphipod species are commonly used with marine sediments. Solid phase Microtox® bioassays can be used to assess toxicity to Vibrio fischeri for both freshwater and marine water sediments. In addition, special tests can be performed using zeolite [7] or Ulva lactuca [8] to identify ammonia toxicity in freshwater and marine sediments respectively.
TIE Methodology
A basic Phase I TIE methodology typically includes a(n):
- Initial toxicity assessment
- pH adjustment/filtration test
- pH adjustment/aeration test
- pH adjustment/solid phase extraction (SPE) test
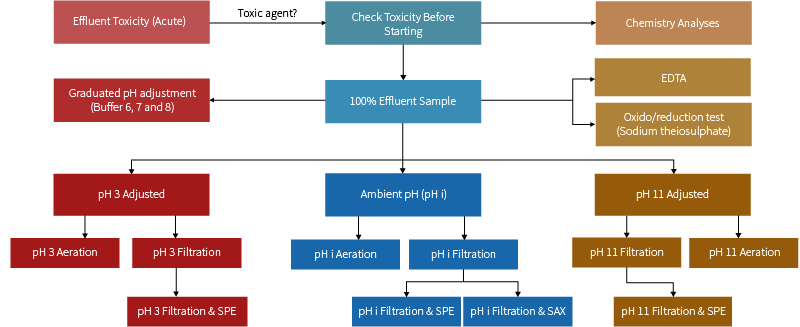
These TIE treatments are summarized in Figure 1.
Figure 1: TIE Treatments
We often find that the pH adjustment filtration, aeration and SPE tests can be used to identify most organic toxicants. The oxidant reduction and EDTA chelation tests are important when inorganic compounds and/or metals are implicated as the agents of toxicity. Each of these tests are described below.
Initial Toxicity Assessment
The purpose of this test is to determine if the sample is toxic. The test is performed on the samples immediately upon receipt and the samples are tested for toxicity using the four bioassays described above:
- Microtox®, bacterial test, Vibrio fischeri, 15 minute assay
- Macroinvertebrate survival, Daphnia magna, 48 hour assay
- Algal growth, Pseudokirschneriella subcapitata, 72 hour assay
- Fish bioassay, fathead minnow, Pimephales promelas, 48 hour assay
Toxicity data (IC50 and LC50) is obtained and converted to toxic units. The data is then reviewed and the most sensitive species identified. This species is then used for the subsequent toxicity tests.
pH Adjustment/Filtration Test
This test provides information on toxicity associated with filterable material. Samples are filtered after pH adjustment (pH 3, 11 and ambient) and the resulting filtrate is adjusted back to ambient pH where necessary and tested for toxicity. By filtering pH adjusted aliquots of samples, those compounds typically in solution at unadjusted pH (i.e. ambient pH) are rendered insoluble or are associated with particles at more extreme pHs and are removed during the filtration process. For example, toxic metals can form insoluble complexes at higher pH and are subsequently removed during filtration, thus rendering the sample less toxic. pH adjustment is performed by adding dilute acid (0.1 N HCl) and dilute base (0.1 N NaOH). In combination these form salt (NaCl), which can be toxic to freshwater organisms and macroinvertebrates. In order to monitor for artificially introduced toxicity, such as salt production during acid/base additions, a control sample consisting of bioassay nutrient water is run in parallel along with the samples. Any toxicity observed for the control sample is then applied to the results obtained for the samples.
pH Adjustment/Aeration Test
Toxic samples are aerated for one hour after pH adjustment (pH 3, 11 and ambient). After sparging, the samples are adjusted back to ambient pH where necessary and toxicity tested. The objective is to determine if volatile constituents are responsible for the observed toxicity. pH adjustment is necessary because some toxic compounds are in various forms depending upon pH. For example, small molecular weight organic acids will be effectively removed by sparging at acidic pH (i.e. pH 3), however, these compounds may be dissociated (or form salts) at higher pH and therefore will not be purged at higher pH.
pH Adjustment/Solid Phase Extraction Test
The pH adjustment/solid phase extraction test is designed to determine the extent of the toxicity caused by organic compounds and metal chelates that are effectively removed by solid phase extraction. The sample is passed through a small column packed with octadecyl‐coated silica (‐SiC18H37) after pH adjustment (pH 3, 9 and ambient). The resulting post‐column effluent is collected, adjusted to ambient pH where necessary and subjected to toxicity testing. If significant toxicity is removed by solid phase extraction, then it is assumed that the toxic chemicals are contained within the solid phase extraction cartridges.
Since these cartridges likely contain many compounds, it is desirable to perform a chromatographic separation followed by toxicity testing in order to isolate the toxic chemicals from non‐toxic chemicals. This is performed by using reverse‐phase chromatography, i.e. the cartridges are stripped with small amounts (4 mL) of water-methanol solutions, including:
- 75% water : 25% methanol
- 50% water : 50% methanol
- 25% water : 75% methanol
- 100% methanol
In reverse‐phase chromatography, chemicals are adsorbed onto the surface of octadecyl‐coated silica. Separation of chemicals is based on polarity or water solubility. Highly polar compounds such as alcohols, carboxylic acids, etc. can be removed by eluting with small amounts of methanol in water (e.g. 75% water : 25% methanol). Compounds that are not polar or hydrophobic require pure methanol to remove them from the column. For example, hydrocarbons such as polycyclic aromatic hydrocarbons (PAHs) require pure methanol in order to strip them from the columns. Compounds with intermediate polarity, such as phenols, amines, ketones, etc. require methanol concentrations of 50‐75% in order to be removed from the octadecyl‐coated silica.
An aliquot of each of the column eluates is diluted with control water and submitted for toxicity testing. The remaining aliquot for each of the column eluates is archived for possible chemical analysis. Dilution with control water is necessary in order to eliminate the toxic effects of methanol. This dilution does not compromise the toxicity data. For example, if a 1000 mL sample is subjected to solid phase extraction and the solid phase cartridge is eluted with 4 mL of water-methanol, the net effect is a 250‐fold concentration. Dilution of 3 mL of the column eluate with 200 mL of nutrient water results in a net 3.75‐fold concentration of the sample. In other words, the toxic effects of methanol can be mitigated without diluting out the toxic effects of the chemicals which caused toxicity in the sample.
Oxidant Reduction Test
This test is designed to determine the role of oxidants such as chlorine in toxicity. The test involves the addition of sodium thiosulphate (Na2S2O3) to effluent samples which will neutralize the toxicity of chlorine and other disinfectant compounds such as ozone and chlorine dioxide. Although originally designed to determine the role of oxidants in toxicity, sodium thiosulphate may also complex with metals such as cadmium, copper, silver and mercury.
EDTA Chelation Test
The EDTA chelation test is used to determine the extent of toxicity caused by cationic metals. Increasing amounts of a chelating agent (EDTA; ethylenediaminetetraacetate ligand) are added to aliquots of the effluent sample. EDTA is a strong chelating agent, and will form relatively non‐toxic complexes with many metals. The success of this treatment depends on several factors including: solution pH, type and speciation of metals, presence of other ligands in solution, and the binding affinity of EDTA for the metal which causes toxicity. The cations most typically chelated by EDTA are aluminum, barium, cadmium, cobalt, copper, iron, lead, manganese, nickel, strontium, and zinc. Selenides, chromates and hydrochromates are not chelated by EDTA, while arsenic and mercury form weak chelates with EDTA.
Complete Inorganic Scan
The inorganic analyses required include: dissolved metals including arsenic, selenium, mercury and antimony. Also included are routine water parameters such as pH, electrical conductivity, calcium, magnesium, sodium, potassium, iron, sulfate, chloride, nitrate/nitrite, total alkalinity, hardness, ammonia, total Kjeldahl nitrogen, total organic carbon, hydrogen sulfide, and dissolved solids. Inorganic analyses are required to determine if chemicals such as metals, ammonia or hydrogen sulfide, salt, etc. are responsible for the observed toxicity.
Organic Analyses of Toxic Fractions
For this particular study, solid phase extraction may be effective at removing toxicity from the samples. Furthermore, toxicants may be effectively isolated by eluting the solid phase extraction cartridges with water: methanol mixtures. Certain water: methanol mixtures may be found to effectively remove toxicants from the solid phase extraction cartridges. These mixtures will be analysed in order to identify the organic compounds responsible for the observed toxicity.
The archived eluate (water-methanol mixture, 1.0 mL) which is found to be toxic is processed as follows:
- 200 μl retained for liquid chromatography/mass spectrometry analysis (LC/MS)
- 800 μl exchanged into chloroform
- 200 μl of the chloroform extract analysed by gas chromatography/mass spectrometry (GC/MS)
- 200 μl of the chloroform extract derivatised with acetic anhydride and analysed by GC/MS
- 200 μl of the chloroform extract derivatised with diazomethane and analysed by GC/MS
- Data obtained from the three GC/MS analyses as well as data obtained from the LC/MS analyses is interpreted, analysed and a list of potential toxicants developed.
In the event that the pH adjustment/aeration test results in significant toxicity reductions, then techniques such as purge and trap gas chromatography/mass spectrometry are applied to identify the toxicants. This technique is applied to compounds with elevated vapour pressures and which can be effectively removed by sparging with air.
Special Methods for Freshwater and Marine Sediments
Identification of Ammonia Toxicity in Freshwater Sediments
Zeolite has been used to identify ammonia toxicity in fresh water sediments. The addition of granulated zeolite to sediments has been shown to reduce the toxicity of ammonia to fresh water invertebrates [7]. Zeolite has been found to be non‐toxic to test organisms and can also be used to treat freshwater and effluent samples to remove ammonia.
Identification of Ammonia Toxicity in Marine Sediments
Ulva lactuca may be used if pH dependent toxicity is noted in the results of the pH adjustment test. This is a new and exciting addition to the TIE protocol for marine water and sediments. Ulva lactuca (sea lettuce) has been used recently to remove ammonia toxicity from marine sediment and water samples for the purposes of toxicity identification [8]. Ulva lactuca has the ability to take up, store and use large amounts of ammonia. This sea lettuce also can take up small amounts of metals present and can absorb organic compounds such as pesticides and PAHs. It can be effectively used in conjunction with the other TIE procedures to identify the toxic constituents in sediment or seawater samples. Ulva lactuca can be obtained commercially from California for purposes of these tests. Five gram pieces of Ulva can be incorporated into bioassay test vessels with the test organisms. It can be a useful alternative to the pH adjustment and aeration tests.
Custom Treatment Test Methods
Bureau Veritas has developed and adapted treatment test methods custom tailored to identify specific suspect compounds in an effluent sample. For example, we have developed a method to selectively remove total suspended solids (TSS) from mine effluents to examine their role in toxicity. We have also used ion selective resins to examine the role of boron toxicity in slag mill effluent. Similarly ultra-filtration and electrophoretic techniques have been applied to examine the role of organic molecules with high molecular weight in pulp and paper effluent samples.
Bureau Veritas is uniquely capable of providing custom treatment tests. We have a collection of experts in the fields of environmental and aquatic toxicology, analytical and process chemistry, acid mine/rock drainage (AMD/ARD), extractive metallurgy, bioleaching, and wastewater treatment technology, all under one roof.
Working with Bureau Veritas
The multidisciplinary nature of our company comprises a team of scientists with many years of experience and expertise in solving problems related to pulp and paper, mining, wastewater treatment, and other industrial effluents.
Bureau Veritas provides TIE and TRE services to customers who need to determine the toxic components in a chemical mixture, such as effluent. We have three ecotoxicology laboratories located in Alberta, British Columbia and Quebec.
References
[1] USEPA (1991). Methods for Aquatic Toxicity Identification Evaluations: Phase I Toxicity Characterisation Procedures. EPA/600/6‐91/003.
[2] USEPA (1993). Methods for Aquatic Toxicity Identification Evaluations: Phase II Toxicity Identification Procedures for Samples Exhibiting Acute and Chronic Toxicity. EPA‐600/R‐92/080.
[3] USEPA (1993). Methods for Aquatic Toxicity Identification Evaluations: Phase III Toxicity Confirmation Procedures for Samples Exhibiting Acute and Chronic Toxicity. EPA‐600/R‐92/081.
[4] USEPA (1999). Toxicity Reduction Evaluation Guidance for Municipal Wastewater Treatment Plants. EPA 833‐B‐99‐002.
[5] Environment Canada, National Contaminated Sites Remediation Program (1992). A Review of Whole Organism Bioassays for Assessing the Quality of Soil, Freshwater Sediment and Freshwater in Canada. Available from Environment Canada, National Contaminated Sites Remediation Program, Ottawa, Ontario, 294 pp.
[6] USEPA (1996) Marine Toxicity Identification Evaluation (TIE): Phase I Guidance Document. EPA/600/R‐96/054.
[7] Besser, J.M., C.G. Ingersoll, E.N. Leonard and D.R. Mount (1998). Effect of zeolite on toxicity of ammonia in freshwater sediments: Implications for toxicity identification evaluation procedures. Environmental Toxicology and Chemistry: 17, 11, 2310‐2317.
[8] Ho, K.T., A. Kuhn, M.C. Pelletier, R.M. Burgess, and A. Helmstetter (1999). Use of Ulva lactuca to distinguish pH‐dependent toxicants in marine waters and sediments. Environmental Toxicology and Chemistry: 18, 2, 207‐212.