Laboratory
PFAS: The Determination of Total Oxidizable Precursors (TOPs)
Oct. 13 2018
While PFAS can be released into the environment directly, there is evidence [1] in the literature to suggest that some may also be formed in situ from the conversion of “precursor” compounds that either already exist in the environment as contaminants, or are co-released with the target PFAS.
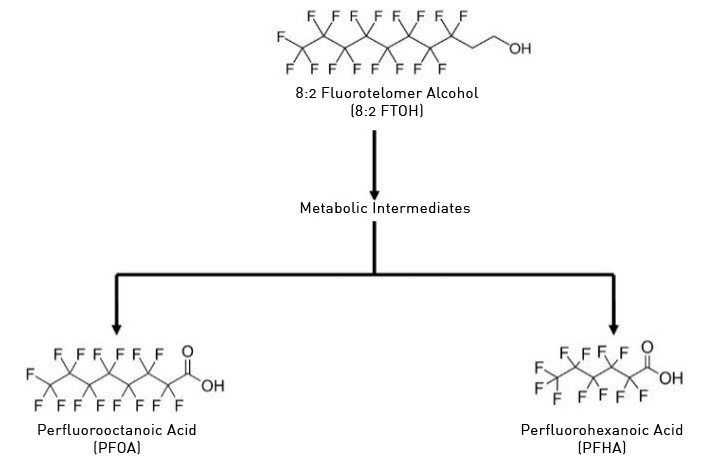
Figure 1: Transformation of 8:2 Fluorotelomer Alcohol (8:2 FTOH)
Common PFAS precursor compounds include fluorotelomer alcohols and fluorotelomer sulfonates. Precursor transformation to PFAS “end products” has important implications for PFAS remediation efforts. By solely focusing on target PFAS removal, without consideration of the total precursor pool, an unanticipated increase in the concentrations of target PFAS may occur over time, resulting in potential future liability.
TOPs Assay
When testing routine samples for PFAS, Bureau Veritas currently reports results for a comprehensive list of known PFAS end products and precursor compounds, in a diverse range of environmental matrices. However, this single test does not measure the potential for PFAS formation due to transformation of precursor compounds over time to the regulated end products. One approach to measure potential PFAS formation in water and soil is to apply the total oxidizable precursors (TOPs) assay [2].
Analytical Method Summary
Bureau Veritas offers the TOPs assay on a variety of matrices, including: surface water; groundwater; wastewater; drinking water; and soils.
The TOPs assay requires that samples be collected in duplicate. One part of the duplicate sample is analyzed for PFAS as received, to establish the initial concentrations of the end products of concern (e.g. PFOS, PFOA, etc.). The second part of the duplicate sample is heated with an oxidizing agent under alkaline conditions to transform any precursor compounds into PFAS end products, and is subsequently analyzed for the routine list of PFAS. The difference between the pre- and post-oxidation concentrations of PFAS represents the presence and potential in situ transformation of poly- and perfluorinated precursor compounds.
Quantitative analyses of the target list PFAS and precursor compounds is performed by using isotope dilution liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS).
The results of the TOPs assay provides a good indication of poly- and perfluorinated materials in a sample, not necessarily measured as part of the routine parameter list, that may transform to the more persistent end products, thereby alerting the data user of potential future liability.
Laboratory Accreditation
Bureau Veritas is accredited by the Standards Council of Canada (SCC), the US National Environmental Laboratory Accreditation Program (NELAP) and the US Department of Defense Environmental Laboratory Accreditation Program (DoD-ELAP) for the analysis of PFAS in environmental matrices.
Reported Parameters
For the TOPs assay Bureau Veritas reports initial and oxidized concentrations for the 25 PFAS as well as the calculated “difference”.
Sample Containers/Hold Times
Water
Samples should be collected in high density polyethylene (HDPE) bottles, provided by the laboratory, and fitted with an unlined (Teflon-free) polypropylene screw cap.
A minimum of 250 mL (2 x 125 mL) of sample is required. The sample hold time is 14 days with proper storage (1-6° C, minimum exposure to light).
Soil
Samples should be collected in high density polyethylene (HDPE) wide-mouth bottles, provided by the laboratory and fitted with an unlined (Teflon-free), polypropylene screw cap. A minimum of 50 g of sample is required. In the absence of any regulated sample hold time, Bureau Veritas adheres to a 28 day hold time for solids with proper storage (1-6° C, minimum exposure to light).
Because of the ubiquitous nature of PFAS compounds in many modern materials, all batches (lots) of sample containers provided by Bureau Veritas, used for collecting samples for PFAS determinations, are “proofed” by the laboratory to demonstrate that they are PFAS-free. Similarly, water used in the field to generate quality control (QC) samples should be PFAS-free.
For a nominal fee, Bureau Veritas will provide PFAS-free water which has been “proofed” by the laboratory.
Analytical Turnaround Time (TAT)
Standard TAT: 15 business days.
Priority TAT: By pre-arrangement only.
Pricing
| WATER | SOIL |
|---|---|
| $1,200/sample | $1,200/sample |
Analytical Limitations
The results of the TOPs assay provide an indication of per- and polyfluorinated materials in a sample that may transform to the more persistent end products. However, it is not without limitations that need to be considered when interpreting the results:
- The number of potential precursor compounds in a sample may be significantly higher than the nine (9) PFAS compounds routinely measured as part of the test. Therefore, not all of the PFAS end products produced by the TOPs assay necessarily result from the oxidation of the nine precursors measured as part of the analysis. Moreover, calculating the increase in end product concentrations may only represent part of the full scope of the precursors present in the sample since the assay only applies to compounds that are oxidizable via this method.
- The oxidation of precursor compounds (known and unknown) may result in intermediate fluorinated compounds or other PFAS that are not measured as part of the analysis. Thus measuring increases or decreases in only the 16 routine PFAS may only represent a portion of the potentially transformable precursors in the sample.
- Complete oxidation cannot be confirmed beyond the nine (9) precursor compounds measured as part of Bureau Veritas’ routine target compound list. Therefore, the TOPs assay may only represent the minimum potential PFAS transformation.
References
[1] Wang, et. al. 2005 Environ. Sci. Technol., 39, 7516-7528.
[2] Houtz, E.F. and Sedlak, D.L. (2012), Environ. Sci. Technol., 46, 9342-9349