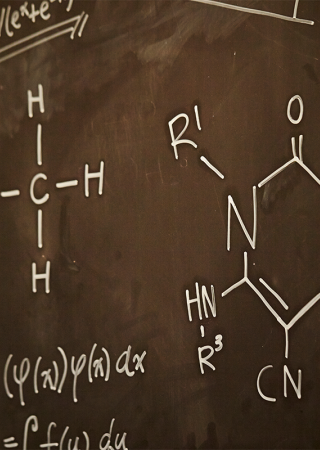
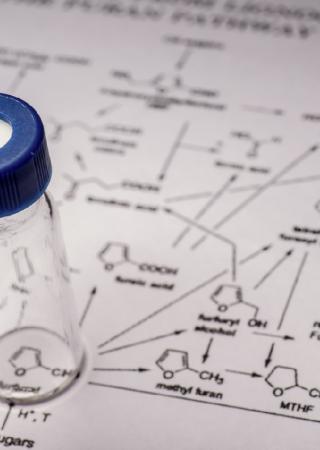
Method Development and Validation
The Bureau Veritas Industrial Hygiene laboratory offers the most advanced instrumentation technologies in High Pressure Chromatography (HPLC), Mass Spectrometry, elemental analysis, optical analysis, and Ion Chromatography. We employ more than 80 professional staff, including Certified Industrial Hygienists (CIH), chemists (Ph.D., MS, and BS levels), and microscopists. We have developed more than 1,000 sampling and analytical methods over the last two decades.
While many of the methods are proprietary, some have been published in peer-reviewed journals, such as the Journal of Occupational and Environmental Hygiene (JOEH) and Journal of Oncology Pharmacy Practice. Our methods are developed in accordance with guidelines from OSHA, NIOSH, and the US EPA, and also meet requirements for US or international product registration.
Following the applicable guidelines, protocols can be customized to meet your project validation requirements. We also provide validation services on existing methods including method transfer, verification and storage stability. Our experts can remediate existing methods to bring those techniques up to current practices. Assuming you do not have a defined method validation protocol for new pharmaceutical application methods, we suggest the following protocols to define scope and validation criteria:
- HPLC and HPLC/MS methods
- Adaptation of HPLC to HPLC/MS to enhance sensitivity
- Adaptation of air sampling methods to surface applications
Method Development and Validation
Download