Laboratory
Validating Air Sampling Methods
May. 20 2019
How method validation works, and why it works to connect new business options with worker safety
In the constant search for new and better products, industrial firms are intensely focused on the development and use of new compounds. “Better living through chemistry” isn’t merely a pop phrase – it describes the continuous exploration of methods for transforming raw materials into substances that unlock new product and process opportunities.
To establish and maintain a position on the forefront of innovation, industrial firms need to be able to move quickly and predictably from invention to production. But speed doesn’t derive strictly from the capacity to formulate new compounds. In order for inventions to be useful in an industrial context, firms need the ability to test for worker and customer safety. In situations involving known or more common chemicals of concern, this testing follows well-established procedures. But when the compound itself is novel, there is a need to innovate in the development of testing processes as well. In this circumstance, industrial firms need the expertise of suppliers who specialize in method validation, which is a documented evaluation of an analytical method that provides assurance that [a new testing procedure] consistently yields results that are accurate and reproducible within previously-established specifications.
The primary driver of industrial hygiene method validation is usually employee safety. Employers need to understand staff risks associated with exposure to new compounds in order to provide appropriate protection and working conditions. They may also need to furnish data on product safety to customers and/or regulators, such as the Occupational Safety and Health Administration (OSHA) and potentially to satisfy concerns of the Environmental Protection Agency (EPA). To demonstrate that risks have been accurately identified and assessed, an accredited laboratory like Bureau Veritas will work through a systematic process, establishing the accuracy and precision performance of a testing method attuned to the characteristics of the target compound.
Steps in the Process – A Case Example
An example of how method validation works in practice is based on experience with a customer who needed validation for a proprietary compound. Due to its chemical and physical properties, the compound might be present as a vapor or an aerosol – in a vapor, liquid, or solid state. That in itself is not a huge challenge, but it does mean that you have to develop a means of collecting the various physical forms of the analyte from the air monitoring environment. To initiate the process, the client provided a sample quantity of the compound and also specified the Occupational Exposure Limit (OEL) that Bureau Veritas would use to determine the appropriate validation range. Our first step was to evaluate commercially-available OSHA Versatile Sampler (OVS) tubes – devices that collect both aerosol and vapor forms of an analyte – to see if they would be appropriate for evaluating the new compound. At this stage, Bureau Veritas tested both the filter used to capture aerosols and the sorbent used to collect the vapor form of the analyte.
In this case, the sorbent material met requirements, but the filter – made of glass fiber – did not perform adequately. This prompted Bureau Veritas to experiment with other filter materials, including nylon and Teflon™. Tests showed that the analyte was retained on the nylon filter under simulated air sampling conditions, and that these filters also met retention targets for short-term storage. Since OVS tubes with nylon filters were not commercially available, Bureau Veritas commissioned a custom order for tubes suited to the compound.
A Battery of Validation Tests
Once the custom OVS tubes were sourced, Bureau Veritas performed the full battery of method validation studies. This included developing the analytical conditions via high-performance liquid chromatography (HPLC), establishing the appropriate calibration range for the exposure levels of concern, and a precision study in which standard solutions of the analyte (ranging from 1/10th of the OEL to two times the OEL) were used to assess analytical precision performance across the target validation range.
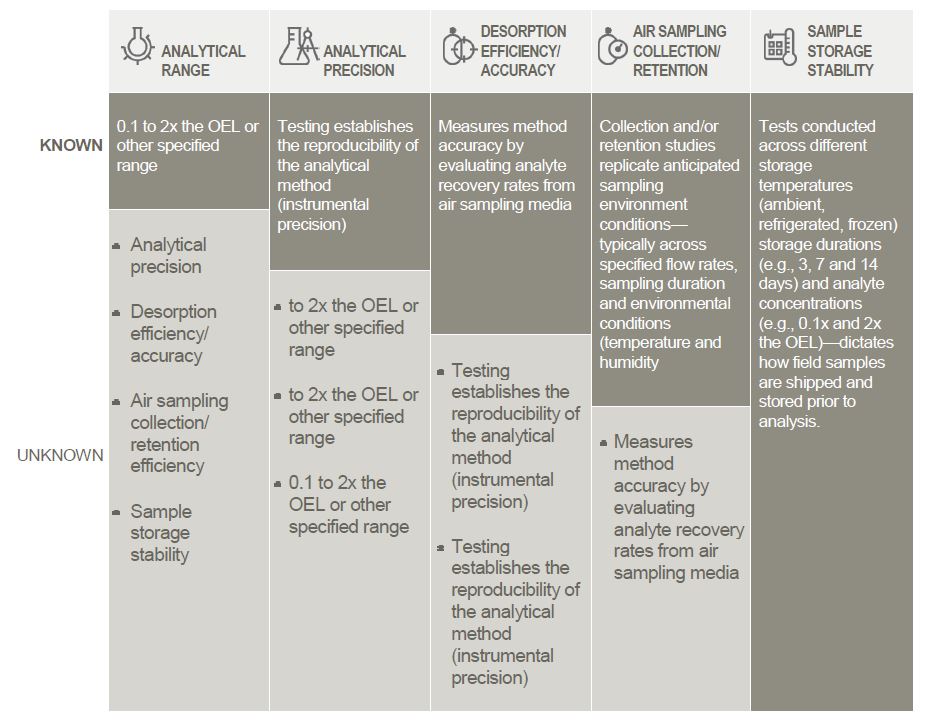
Table 1: Moving to certainty – the Method Validation process
The Method Validation process uses a series of defined steps to ensure that compound testing is accurate and precise – that the testing procedure exhibits acceptable results across multiple studies, and that these results will produce outcomes consistent with regulatory and/or customer requirements. As the figure shown here demonstrates, this is achieved through a series of steps that systematically address defined field sampling scenarios, building to a state where instrument precision, sampling/measurement capabilities and storage stability are fully characterized.
Desorption Efficiency/Accuracy
The next step in the method validation process is testing the air sampling media – the custom OVS tube. This is a two-step process. It starts with a desorption efficiency study; as a general protocol, this would involve evaluating four different mass loading levels (from 0.1x to 2x the OEL) with six replicates per level, though this regimen can be changed to accommodate additional tests or parameters depending on client needs. In this process, the media is spiked with the compound at different levels and then analyzed to determine recovery rates (ideally, 100%, though acceptance ranges according to OSHA/NIOSH are generally 75% - 125% recovery).
Desorption Efficiency Validation Results: 100% over the target validation range from 0.1x to 2x OEL.
Air Sampling Collection/Retention Efficiency
If desorption efficiency performance is acceptable, the next step is to conduct air sampling studies. These studies vary depending on the form of the analyte: volatile substances that will be in vapor form are tested through assessment of collection efficiency on the media, while non-volatile substances (including aerosols and solids) are evaluated via retention efficiency studies. Similar to desorption, retention efficiency studies involve spiking the analyte onto the media followed by air sampling under specific flow, sampling period, and environmental conditions to determine if the analyte is retained on the media. For Time-Weighted-Average (TWA) monitoring, air sampling studies are generally conducted over an eight-hour period to represent a typical work day scenario.
Retention Efficiency Validation Results: 97% over the target validation range from 0.1x to 2x OEL.
The Final Hurdle: Sample Storage Stability
Once acceptable precision and accuracy of the sampling media and analytical technique are established, one issue remains: determination of analyte stability on the media after sampling is complete. Variables evaluated include storage time and temperature. Storage stability deals with questions like ‘can samples be shipped at ambient temperature or do they need to be shipped under cold conditions?’ and ‘what is the recommended hold time between collection and analysis?’
Sample storage stability is usually performed at two mass loading levels – generally, the extremes of validation range (0.1x and 2x the OEL). I recommend that these tests evaluate storage out to 14 days at a minimum, although 30- and 60-day windows are not uncommon. In a typical 14-day test, Bureau Veritas will conduct stability evaluations at 3, 7 and 14 days. Storage temperature conditions vary with what is known about the target substance: non-volatile analytes are often tested under ambient and refrigerated conditions, while testing for volatile substances may also include frozen conditions. I like to see stability for three days at ambient temperature, to provide time for sample collection and forwarding to the laboratory. Note: If you’re evaluating different temperatures and a certain number of days at different time points, you get a full picture of what storage conditions you might need to have onsite and what your sampling and analysis schedule will need to be in order to maintain stability.
Sample Storage Stability Validation Results: Stable for a period up to 14 days under ambient and refrigerated storage conditions.
Key Results
Bureau Veritas was successful in validating an air sampling and analysis method for the client’s compound of interest over a range of 0.1x to 2x the OEL. The chemical/physical properties of the analyte required sampling using both a filter and sorbent material. A custom OVS tube was necessary in order to achieve acceptable retention, recovery, and stability performance on both the filter and sorbent material.
The Bottom Line
In an economic environment that rewards rapid innovation, the ability to quickly and confidently move forward with new inventions is a critical aspect of competitiveness. This requires that firms obtain “scientific data to support the fact that they can collect and monitor for an analyte in the air with known accuracy and precision.” By working with Bureau Veritas on method validation, industries are able to keep pace with competitors and meet the safety needs of their workers and the broader interests of regulators, shareholders and customers.