Laboratory
PFAS Replacement Compounds
Oct. 12 2018
As global production of PFOS and PFOA is eliminated, manufacturers are focused on developing PFAS replacement technologies, including reformulating or substituting longer chain PFAS (e.g. PFOS and PFOA) with shorter-chain perfluoroalkyl or polyfluorinated substances that include, but are not limited to, compounds produced through electrochemical fluorination (ECF) and fluorotelomerization, such as: fluorotelomer alcohols (FTOH), perfluorobutane sulfonyl fluoride (PBSF)-based derivatives (e.g., perfluorobutane sulfonate (PFBS) as a substitute for PFOS) and polyfluoroethers (e.g. GenX, ADONA and F53B used in the manufacture of fluoropolymers) among others.
Studies are suggesting that some of the lower molecular weight PFAS replacement compounds may or more importantly, may not be less hazardous than their long-chain predecessors. Information on the type and extent of environmental contamination by replacement PFAS although is limited, as most are not currently reported as part of the routine suite of PFAS analyzed by contract analytical laboratories.
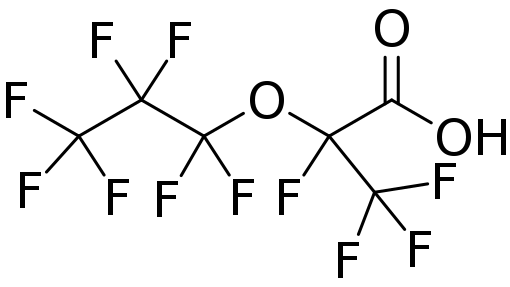
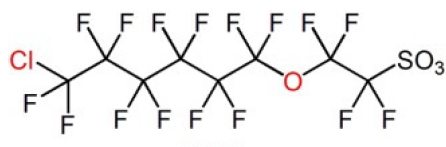
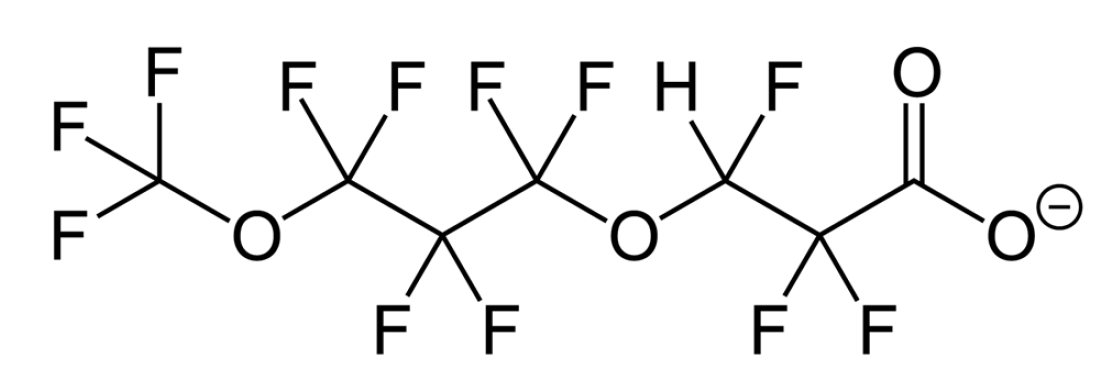
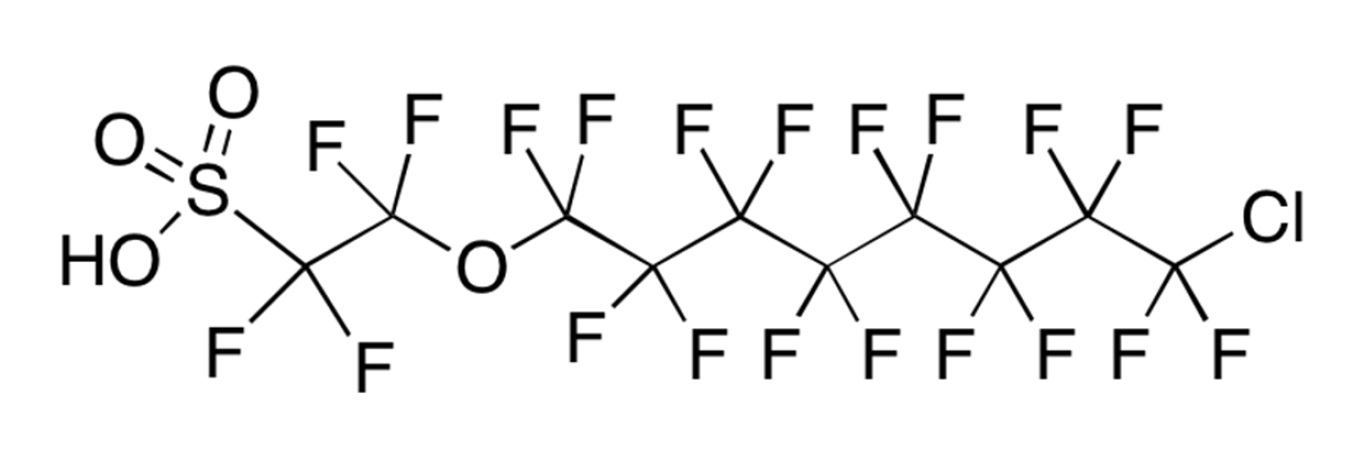
Three key PFAS replacements being studied are: 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid; dodecafluoro-3H-4,8-dioxanoate; and a combination of 9-chlorohexadecafluoro-3-oxanonane-1-sulfonate and 11-chlororeicosafluoro-3-oxaundecane-1-sulfonic acid. These are commonly referred to as GenX, ADONA and F53B, respectively. Replacement chemicals, like GenX, tend to have fewer carbon atoms in the chain, but have many similar physical and chemical properties as their predecessors PFOS and PFOA (e.g. they repel oil and water).
Industries in the United States have phased out production of PFOA and PFOS because of potential health risks to humans and have begun using replacement PFAS, such as GenX. There is a substantial body of knowledge for managing risk from PFOS and PFOA, but much less knowledge about the replacement PFAS.
GenX is actually a brand name for a chemical process that uses 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)propanoic acid and produces 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy) pro-panoate and heptafluoropropyl 1,2,2,2-tetrafluoroethyl ether. Manufactured by Chemours (formerly Dupont), these chemicals are used in food packaging, paints, cleaning products, non-stick coatings, outdoor fabrics and firefighting foam among others. GenX chemicals are used as replace-ments for perfluorooctanoic acid (PFOA) for manufacturing fluoropolymers such as Teflon®.
ADONA is another replacement compound for PFOA.
F53B has been used in China by several producers as replacement PFAS since the late 1970s as wetting agents and mist-suppressing agents in decorative plating and non-decorative hard plating.
Because of the increase in interest in PFAS replacement compounds, Bureau Veritas has validated methods for the determination of GenX, ADONA and F53B by liquid chromatography coupled with mass spectrometry.
Sample Containers/Hold Times
Water
Samples should be collected in high density polyethylene (HDPE) bottles, provided by the laboratory, and fitted with an unlined (no Teflon) polypropylene screw cap. A minimum of 125 mL of sample is required. The sample hold time is 14 days with proper storage (1-6° C, minimum exposure to light). Sample containers should be filled completely, to minimize surface-to-volume ratios and reduce the possibility of biasing results.
In accordance with USEPA Method 537, chlorinated drinking water samples may require preservation with Trizma® at the time of sampling, to quench the effects of residual free chlorine and buffer the sample to a pH of approximately 7.
Soil and Tissue
Samples should be collected in high density polyethylene (HDPE) wide-mouth bottles, provided by the laboratory, and fitted with an unlined (no Teflon), polypropylene screw cap. A minimum of 50 g of sample is required. In the absence of any regulated sample hold time, Bureau Veritas adheres to a 28 day hold time for solids and tissues with proper storage (1-6°C, minimum exposure to light).
All batches (lots) of sample containers provided by Bureau Veritas, used for collecting samples for PFAS determinations, are “proofed” by the laboratory to demonstrate that they are PFAS-free.
Similarly, water used in the field to generate quality control (QC) samples should be PFAS-free. For a nominal fee, Bureau Veritas will provide PFAS-free water which has been “proofed” by the laboratory.
Analytical Method
Bureau Veritas provides analyses for PFAS on a diverse range of environmental matrices, including among others: aqueous film forming foams (AFFFs), drinking water, groundwater, leachate, soil and other solids and tissue.
Low level water samples undergo solid phase extraction (SPE), to extract, clean up and concentrate the parameters of concern. The extract is then analyzed by isotope dilution liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS).
Water samples having higher contaminant concentrations (often requiring dilution) may be analyzed by direct injection isotope dilution LC/MS/MS.
Soil, solids and tissues are homogenized, and then undergo a solid/liquid extraction. Interferences are removed from the liquid extract using solid phase extraction (SPE). The resultant extract is then concentrated and analyzed by isotope dilution LC/MS/MS.
Reported Parameters
Bureau Veritas currently reports up to 32 PFAS and precursor compounds, including 4 next generation replacement compounds. Reporting limits (RLs) and method detection limits (MDLs) for these parameters have been validated at part-per-trillion (ppt).
Analytical Turnaround Time (TAT)
Standard TAT: 10 business days.
Priority TAT: By pre-arrangement only.
Laboratory Accreditation
Bureau Veritas is accredited by the Standards Council of Canada (SCC), the US National Environmental Laboratory Accreditation Program (NELAP) and the US Department of Defense Environmental Laboratory Accreditation Program (DoD-ELAP) for the analysis of PFAS in environmental matrices.